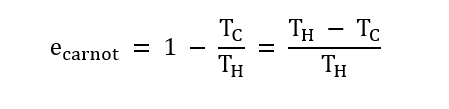
The Second Law of Thermodynamics
There are lots of definitions for the second law. Every single definition is a different approach for a different application. I will give some of the definitions and try to explain these.
One of the definitions states that the total entropy of a closed system can only increase or remains the same. We have not covered the topic entropy but still I felt that I need to give this definition at first because in my opinion it is the most important one. We will cover the entropy and its relationship between the second law in the next section.
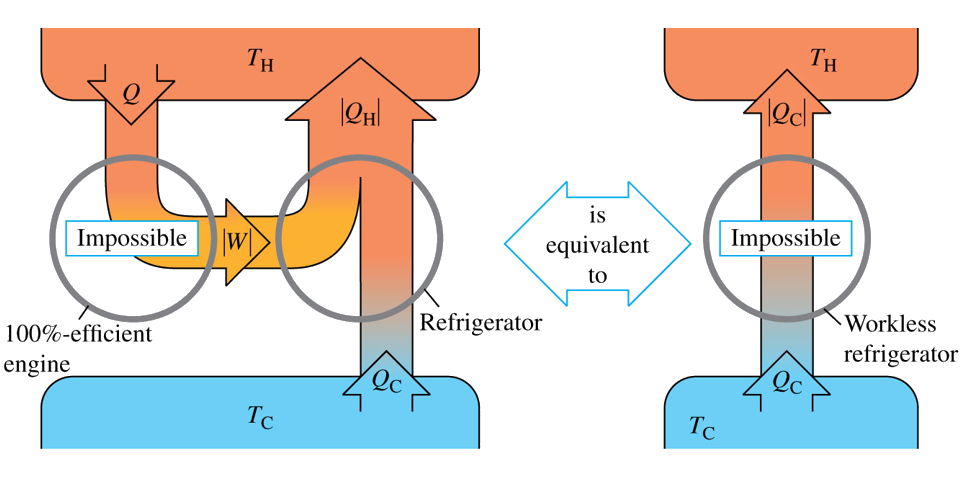
Another definition is known as Kelvin-Planck statement or the engine statement and it states that “It is impossible for any system to undergo a process in which it absorbs heat from a reservoir at a single temperature and converts the heat completely into mechanical work, with the system ending in the same state in which it began."

I should remind you that the second law is not a deduction from the first law but stands by itself as a separate law of nature. As I mentioned at the beginning, there are several events in nature which do not violate the first law but do violate the second. In general, the second law limits the availability of energy and the ways in which it can be converted while the first law denies the possibility of creating and destroying energy. If the second law were not true, we could do so many things such as powering a heat engine with %100 efficiency and that would solve the energy problem of the planet.
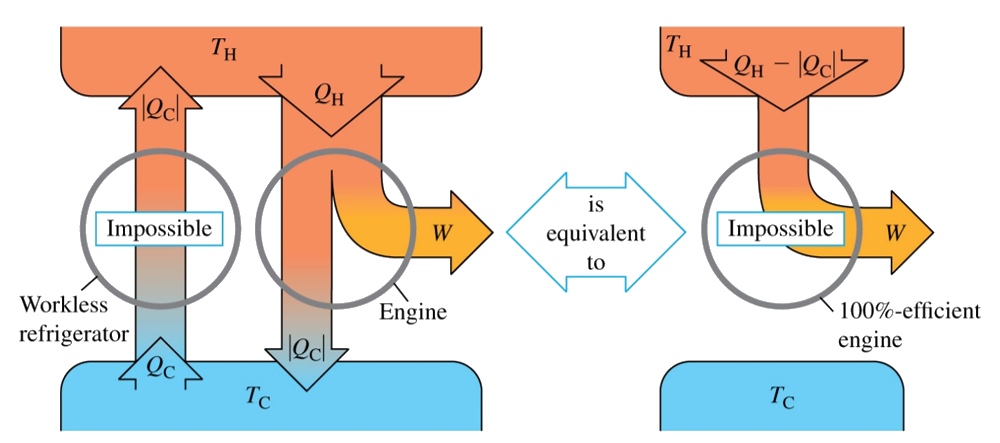
There is also another definition called Clausius statement or the refrigerator statement which states “It is impossible for any process to have as its sole result the transfer of heat from a cooler to hotter body.” As I said before these definitions are all same in principle. They just differ from each other by the way they built and their application area.
The Carnot Cycle
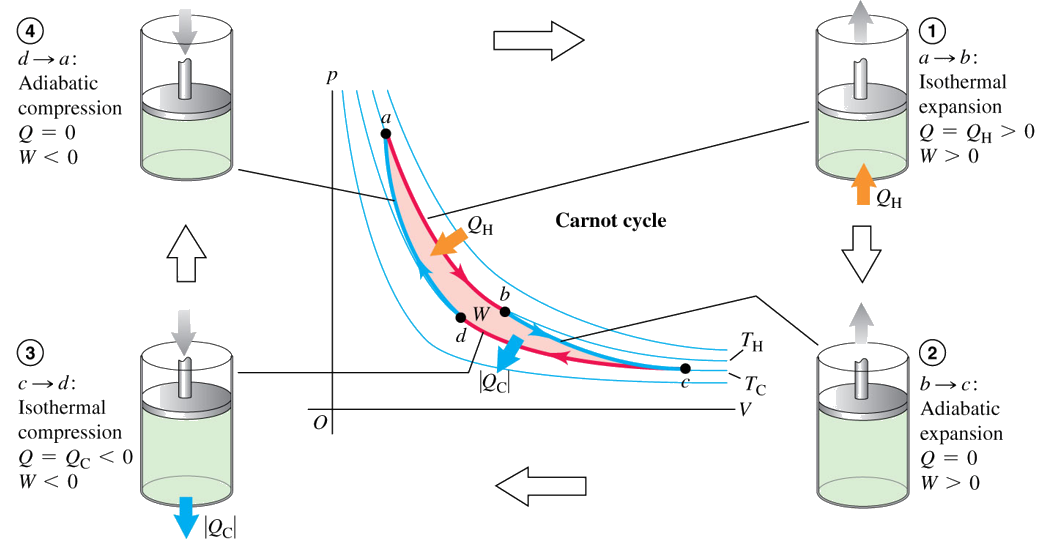
Carnot engine is a hypothetical engine which has the maximum efficiency while obeying the second law. The idea was developed by a French engineer, Sadi Carnot (1796-1832).
The Carnot cycle consists of the following steps:
1. The gas expands isothermally at temperature TH, absorbing heat QH.
2. It expands adiabatically until its temperature drops to TC.
3. It is compressed isothermally at TC, rejecting heat |QC|.
4. It is compressed adiabatically back to its initial state at temperature TH.
The Carnot Cycle and Second Law
It is proved that "no engine can be more efficient than a Carnot engine operating between the same temperatures." It also follows that "all Carnot engines operating between the same two temperatures have the same efficiency, irrespective of the nature of the working substance."
Next Section >>> Entropy