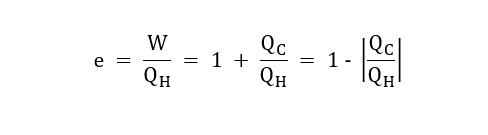.png)
Heat Engines
Heat engine is a device that can convert heat partly into mechanical energy or work. The substance called the working substance is the quantity of matter inside the engine which undergoes inflow and outflow, expansion and compression, and sometimes change of phase.
For analyzing the topic easier, we will take engines which work in cyclic processes into account. These are the kind of engines that leave the working substance in the same state in which it started after a sequence of processes. For example, in a steam turbine the water recycled and used over and over.
.png)
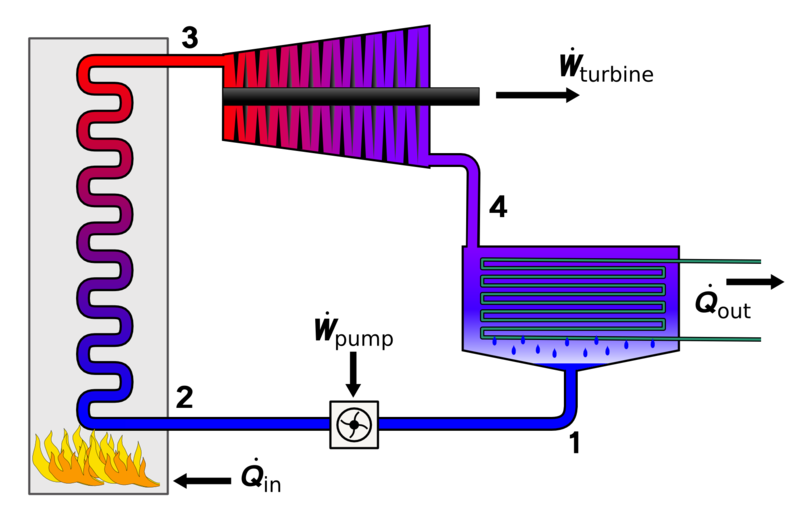
Water in the system undergoes different processes (heating, cooling etc.) but at the end of every cycle it is in its initial condition.
Hot and Cold Reservoirs
The working principle of heat engines is to absorb heat from a source at a relatively high temperature, perform some mechanical work, and discard or reject some heat at a lower temperature. In this case, the discarded heat is wasted.
In a cyclic process, since its initial and final internal energies are equal, due to The First Law of Thermodynamics,
U2 - U1 = 0 = Q - W
Q = W
It can easily be seen that the net heat flowing into the system in a cyclic process is equal to the net work done by the system.
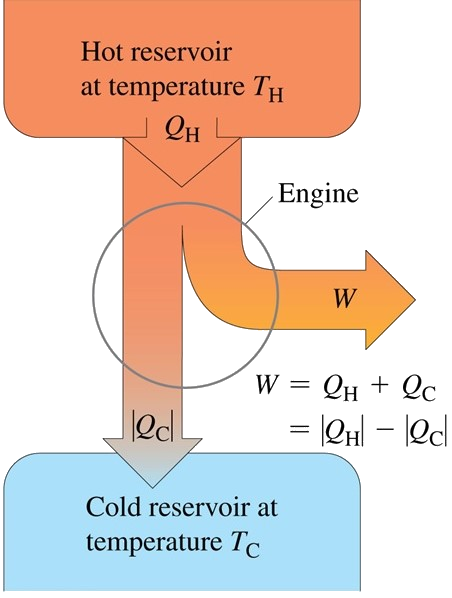
To better understand the heat engine’s working principle, it can be thought that there are two bodies with which the working substance of the engine can interact. The first one is called the hot reservoir, which is the heat source. It is the body which gives the large amount of heat at a constant temperature TH to the working substance without changing its own temperature. The second one is called the cold reservoir. It is the body which absorbs large amounts of discarded heat from the engine at a constant lower temperature TC. Also, we can denote the quantity of heat transferred from the hot reservoir to the cold one as QH, and from the cold one to the hot one as QC. It is obvious that the quantity of heat Q is positive when the working substance gains heat and vice versa.
Energy-Flow Diagrams and Efficiency
Energy transformations in a heat engine can be represented by the energy flow diagram as above. There are proportionalities between QH and the width of the incoming pipeline, and between |QC| and the width of the outgoing pipeline. The line which is to the right represents the portion of the heat supplied that the engine converts to mechanical work W.
The net heat Q absorbed per cycle is,
Q = QH + QC = |QH| - |QC|
The net work is,
W = Q = QH + QC = |QH| - |QC|
Normally, our aim is to convert all the heat into work. However, experiments show that it is impossible. That means, QC is never equal to zero and thus, there is always some heat wasted.
The thermal efficiency of an engine, e,
e = W/QH
So, in general,
That is the end of this section. Since, my aim is to explain the concept entropy and The Second Law of Thermodynamics, I will not go into detail of Heat Engines. If you wonder what are these details, you can check the 'Videos and Readings' section.
Next Section >>> The Second Law of Thermodynamics