Directions of Thermodynamic Processes
Thermodynamic processes, occuring in nature are completely irreversible. These processes can only move in one direction. The term 'irreversible' in this concept means that you cannot change the process' initial and final points. If you could change these, it would not seem natural to your eye. To understand it better, check the example below.
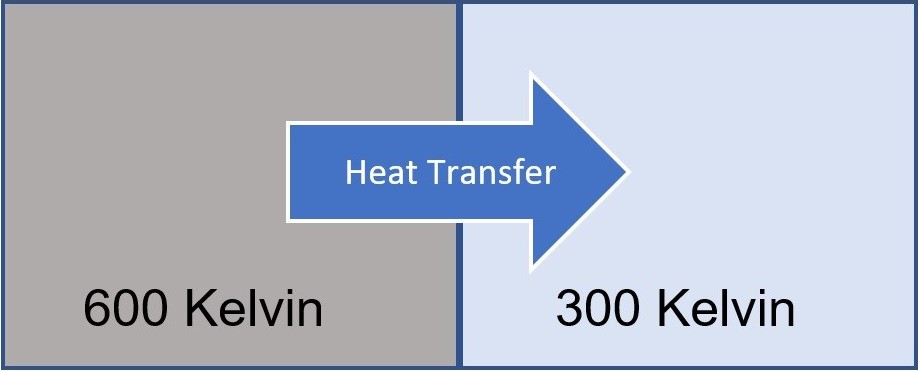
Let's say we have two materials with different temperatures that are touching each other. In this case, we all expect that heat will flow from the hot one to the cold one (Figure I). This process is completely irreversible. No one can observe the heat flowing from cold one to the hot one, which would be a reversible process.

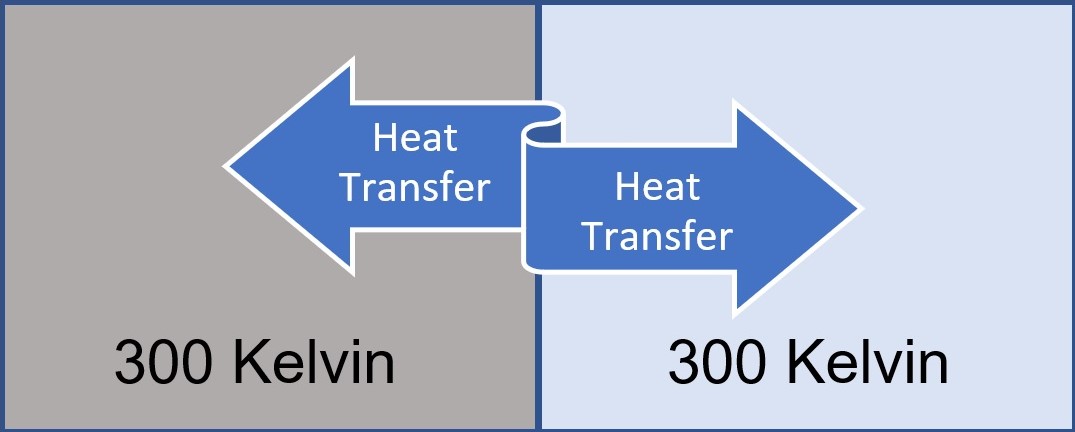
However, we can also consider ideal systems that are reversible. These systems should be in thermal equilibrium with their environment. In this case, any change of state can be reversed by making an infinitisimal change in its conditions. For example, if the temperatures of the two materials that are in contact are very close to each other (Figure II), the direction of the heat flow can be determined by changing the materials’ temperatures infinitesimally.
As a result, reversible systems are equilibrium processes and there should always be a almost thermal equilibrium state. Of course, if the system is completely in thermal equilibrium, there will be no heat flow. Therefore, the system is not completely in the form of thermal equilibrium, but very close to it. Thus, reversible systems are merely an idealization and do not really exist. However, if the appropriate conditions are met, the process can be thought as a reversible process.
Next Section >>> Heat Engines